How Many Valence Electrons in Bromine
Valence electrons in Bromine Br 7. Valence electrons given by bromine atoms 7 2 14.
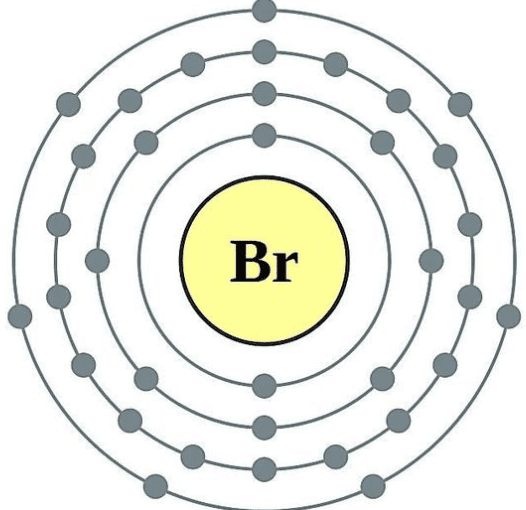
How Many Valence Electrons Does Bromine Have
One two five seven.
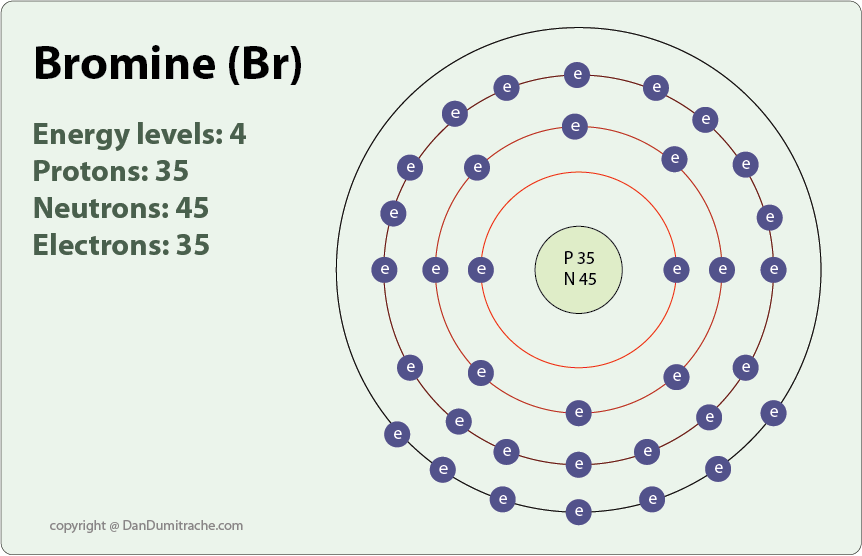
. The how-many-valence-electrons-does-iodine-have have 2020-11-17 135514 and PT1M29S. A valence electron is an outer shell electron and may participate in the formation of a chemical bond. Total valence electrons 14.
The elements that have 5 6 or 7 electrons in the last shell receive the electrons in the last shell during bond formation. Now we just need to count up all of these valence ones. Now lets check the facts about Bromine.
Valence electrons are the electrons present in outermost shell of an electron. Ok but how many valence electrons does an atom of Bromine have. There are two ways to find the number of valence electrons in Bromine Br.
How many valence electrons do chlorine bromine and iodine have OneClass 73 MB Download. For main group elements ie s-block and p-block elements the valence electrons are the electrons present in the outermost orbit. In bromine there are 35 total electrons which means that 17 of them will be valence ones since theyre closer to the nucleus than any other electron.
Valence electrons in Rubidium Rb 1. Bromine Overview Bromine Valence Electrons 1357 Atomic Number 35. Also Know how many stable electrons does bromine have.
The first is to use the Periodic Table to figure out how many electrons Bromine h. Valence electrons found in the s and p orbitals of the highest energy. Bromine belongs to halogen family.
See on Periodic Table. There should be 9. Bromine has 7 valence electrons since it is in group 7 and for electron configuration.
This means a bromine atom has seven valence electrons. Each electron is influenced by the electric fields produced by the positive nuclear charge and the other Z 1 negative electrons in the atom. Therefore the valence electrons of bromine are seven.
Counting the 4th shell orbitals and their electrons Bromine has two 4s electrons and five 4p electrons giving it a total of 7 valence electrons. For example bromine Br is a member of halogen family that is group 17. Valence electrons in Strontium Sr 2.
The number of electrons in an electrically-neutral atom is the same as the number of protons in the nucleus. Valence electrons in. Bromine belongs the group 7 of the periodic table thus it has seven electrons in its outermost shell.
Therefore Bromine has 7 valance electrons. All the elements in the group have the same valence electronic configuration that is 7. Bromine Z35 which has 35 electrons can be found in Period 4 Group VII of the periodic table.
Valence electrons in Krypton Kr 8. At times all these seven valence electrons can be involved in a chemical bonding in order to form an octet structure. Valence shell configuration of bromine is 4s 2 4p5.
Bromine is a group VIIA element in the periodic table and contains seven electrons in its last shell. Therefore the number of electrons in neutral atom of Bromine is 35. The question is asking for the number of valence electron that are available fo bonding in bromine.
In the case of Bromine the valence electrons is 1357. The electron configuration of bromine shows that the last shell of bromine has seven electrons. How many valence electrons does bromine have.
Find an answer to your question How many valence electrons are available for bonding in bromine Br. Bromine has an electron configuration of 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 1 0 4 p 5 the valence electrons are in the 4 s and 4 p orbitals giving Bromine 7 valence electrons. Details of How to Find the Valence Electrons for Iodine I MP3 check it out.
Since bromine has 7 valence electrons the 4s orbital will be completely filled with 2 electrons and the remaining five electrons will occupy the 4p orbital. Now we know how many electrons are there in valence shells of bromine atoms.

How Many Valence Electrons Does Bromine Br Have
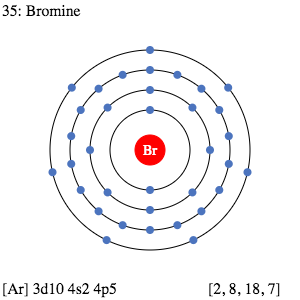
Bromine Valence Electrons Bromine Valency Br Dot Diagram

Bromine Br Electron Configuration And Orbital Diagram

Why Does Fluorine Have A Higher Ionization Energy Than Bromine Socratic
No comments for "How Many Valence Electrons in Bromine"
Post a Comment